Education
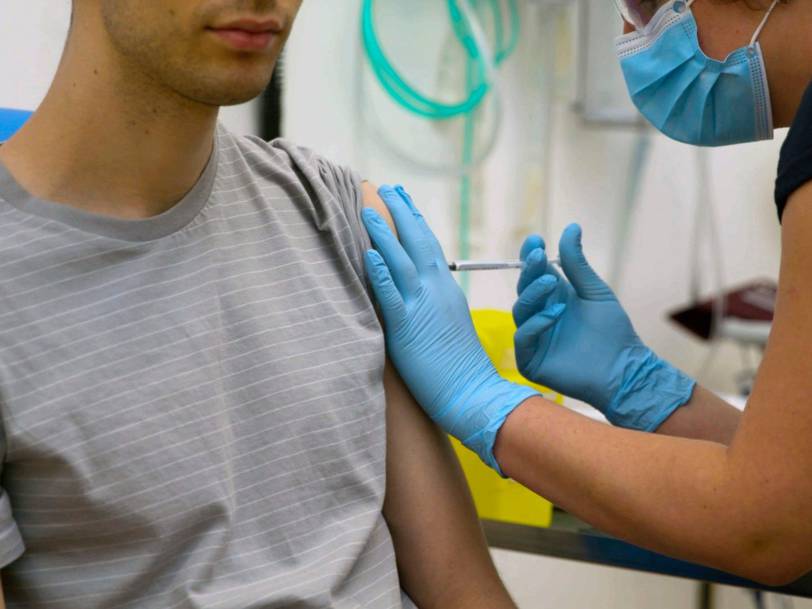
A Chinese biotech company has just published the first human data for its coronavirus vaccine candidate, supporting further studies

The experimental vaccine is currently being tested in a larger, mid-stage trial. Researchers have indicated that the early findings of 108 people support further study.
The experimental coronavirus vaccine developed by the Chinese biotech company became the first candidate to publish human data in a medical journal.
Researchers who tested CanSino Biologic in 108 volunteers in Wuhan , China, published their findings Friday in The Lancet, a leading medical journal.
Scientists say that the vaccine was well tolerated and that early data support additional trials. The CanSino vaccine is now being tested in a larger, mid-stage trial.
Almost all volunteers developed some amount of antibodies and T-cells, essential components of the immune system's response to invasive pathogens. However, it is not yet clear to scientists what levels of immune response are needed to protect people from infection.
Approximately 4 in 5 participants had some level of side effects. Nine people experienced extreme fevers, but the researchers reported that all side-effects were self-resolving and lasted no longer than two days.
One of the leading candidates for coronavirus vaccine showed signs of promise in an early human health report.
CanSino Biologic vaccine was tested in 108 healthy volunteers at three doses: low , medium and high. Researchers concluded that it was tolerable and led to immune responses, which supported further clinical trials. The data was published in The Lancet, the top medical journal, on Friday.
These are the first published data from the coronavirus vaccine program. Massachusetts biotech Moderna published a favorable summary of the preliminary findings earlier this week, but did not include details or publish the information in a medical journal. Moderna said the data is in the hands of the US National Institutes of Health, which will release it at a later date.
The vast majority of participants — about 4 in 5—recorded some level of side effects, usually pain around the injection site (54 per cent reported), fever (46 per cent), fatigue (44 per cent) and headache (39 per cent). Within one month of vaccination, none of the 108 volunteers had any serious adverse events, the researchers found.
Nine people had a "case of high fever," the researchers said. Five of these individuals were in a high-dose group , which means that 14 per cent of the high-dose population had extreme fever. In total, 17 percent of the high-dose group had some form of serious side effect.
There were also self-resolving side effects, which, according to the results, lasted no more than 2 days after injection.
While the study was primarily aimed at ensuring that the vaccine was safe in humans, researchers also measured antibody levels in vaccinated volunteers. Antibodies are virus-fighting proteins that play a vital role in the prevention of potential viral infections.
Researchers tested for two types of antibodies: binding and neutralising. While binding antibodies will take hold of the virus, they do not necessarily prevent it from infecting and replicating healthy cells.
There were also self-resolving side effects, which, according to the results, lasted no more than 2 days after injection.
While the study was primarily aimed at ensuring that the vaccine was safe in humans, researchers also measured antibody levels in vaccinated volunteers. Antibodies are virus-fighting proteins that play a vital role in the prevention of potential viral infections.
Researchers tested for two types of antibodies: binding and neutralising. While binding antibodies will take hold of the virus, they do not necessarily prevent it from infecting and replicating healthy cells.
Neutralizing antibodies are a vital measure for the vaccine, because they can help destroy the virus and avoid infection.
What amount of neutralizing antibodies people need to fend off infection remains uncertain. Scientists are actively seeking to find out why by observing the antibody responses of COVID-19 patients recovered, as well as by researching animals such as rats, guinea pigs and monkeys.
"Neutralizing antibodies to live SARS-CoV-2 were all negative at day 0 and increased moderately at day 14, peaking at 28 days post-vaccination," the researchers wrote.
They found that the concentration of neutralizing antibodies increased along with the dose strength.
Nearly all participants had substantial rises in the amount of binding antibodies. Almost 60 per cent had at least a four-fold increase in neutralizing antibodies.
Another critical component of the immune response was also measured by the researchers: T-cells. Detectable levels of T-cells were found to peak after two weeks. "High proportions of participants with positive T-cell responses were observed across all post-vaccination dose groups," they wrote.
The amount of antibodies and T-cells required to protect humans from infection remains unclear.
